Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS) is a method for surface analysis of materials that enables the investigation of complex chemical compounds. For this purpose, the material is bombarded with primary ions (Bi+, Bi3 +), whereupon the atoms and molecules on the surface ionize: By adding or removing one or more electrons, they become charged particles (secondary ions), which can then be detected using time-of-flight analysis. Lighter secondary ions reach the detector faster, heavier secondary ions arrive later. The mass of the ions and therefore the chemical composition of the material can be deduced from the time of impact. Each chemical substance has a unique fingerprint, which can in principle be used for identification.
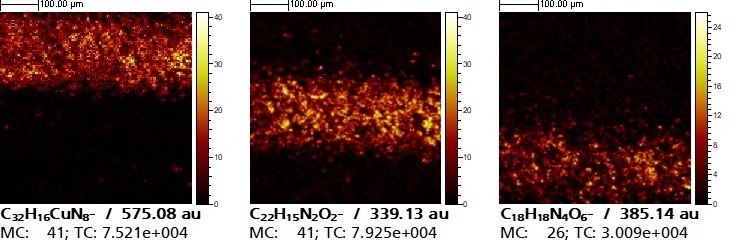
In the resulting surface spectrum, mass peaks of the elements and molecules and their fragments (caused by the bombardment with primary ions) can be recognized. In addition, there are all combinations of possible isotope signals and also molecular compounds that only arise as a result of the bombardment. Due to the high number of possible fragmentations and possible chemical compounds, identification is only possible if reference data (e.g. from a database) or additional information is available.
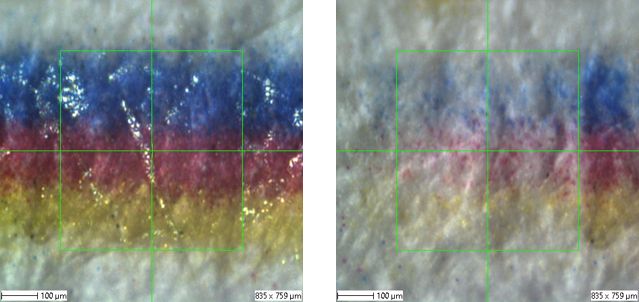
To demonstrate the efficiency of the method, paper samples printed with stripes in the primary colors cyan, magenta and yellow were examined at the Fraunhofer IMWS. The lines were chosen to be as thin as possible so that they fit into a typical measuring field of 500 µm x 500 µm for ToF-SIMS surface analysis.
There is a passivation on the printed color lines that protects direct access to the color pigment surface - and initially blocks it for surface analysis. In order to obtain a surface spectrum of the color pigments, the passivation must first be removed using a suitable method without destroying the chemical compound of the color pigments underneath the passivation. A gas cluster ion source was used for this purpose. This uses Ar clusters consisting of clusters with a high number of Ar atoms (>1500). As the Ar clusters are comparatively large in relation to the energy, the organic passivation layer can be removed without destroying the chemical compounds underneath.